Carbohydrates
Image from http://www.layevangelism.com/bastxbk/images/deoxyribose.jpg
-most common organic compounds (biomolecules)
-are energy sources for cells - broken down by cellular respiration (needs O2)-17kJ/g, half as much as lipids, but breakdown is much faster
-C,H,O
-can be mono-, di- and polyssacharides
a. Monosaccharides
-sweet, H2O soluble, crystalizable
-general formula: (CH2O)n
Triose sugars (n=3)
-these are all intermediate products (köztes termékek) in biochemical reactions
-most common: glyceraldehyde (oxidation product of glycerol)
Pentose sugars (n=5)
-biologically important: parts of nucleotides and nucleic acids, most common are deoxyribose and riboseImage from http://www.layevangelism.com/bastxbk/images/deoxyribose.jpg
Hexose sugars (n=6)
-most common monosaccharides, eg. alpha-glucose, beta-glucose (szölőcukor), fructose (gyümölcscukor) and galactose are all isomers of C6H12O6 and form the monomers of most di- and polysaccharides
Image from http://www.nzetc.org/etexts/Bio14Tuat01/Bio14Tuat01_036a%28h280%29.jpg
Image from http://www.eastchester.k12.ny.us/schools/hs/teachers/fishman/images/fructose_000.gif
Image from http://www.eastchester.k12.ny.us/schools/hs/teachers/fishman/images/fructose_000.gif
Galactose
Image from https://teach.lanecc.edu/naylore/225Lectures/04A/StructureImages/2galactoseThm.jpg
b. Disaccharides
-2 monosaccharides combine by condensation to form a disaccharide
Examples:
glucose + fructose = sucrose (table sugar) - remember this is the main form in which carbohydrates are transported in plants!
Image from http://www.pharmas.co.uk/blog/wp-content/uploads/2009/04/glucose-fructose-and-sucrose.jpg
glucose + fructose = sucrose (table sugar) - remember this is the main form in which carbohydrates are transported in plants!
Image from http://www.pharmas.co.uk/blog/wp-content/uploads/2009/04/glucose-fructose-and-sucrose.jpg
2 alpha glucoses = maltose - common in germinating seeds, result of hydrolysis (break down) of starch
Image from http://www.eastchester.k12.ny.us/schools/hs/teachers/fishman/images/maltose.gif
Image from http://www.eastchester.k12.ny.us/schools/hs/teachers/fishman/images/maltose.gif
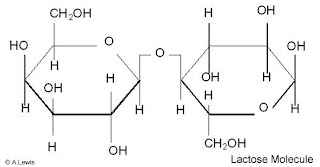
glucose + galactose = lactose (tejcukor) - found in milk
Image from http://dspace.dial.pipex.com/town/park/gfm11/nomilkgif/LACTOSE.JPGc. Polysaccharides
- largest carbohydrates
- long chains of monsaccharides (hexose sugars)
- can be folded
- general formula: (C6H10O5)n
- broken down into monomers (by hydrolysis) if needed for energy
- insoluble in H2O because of size
- not sweet
- cannot crystalize
TYPES of polysaccharides:
i. Storage polysaccharides
-insoluble, therefore they won't diffuse out of cells and won't affect osmosis
Examples:
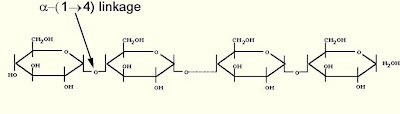
STARCH: found in plants - made of 2 types of chains
STARCH: found in plants - made of 2 types of chains
-amylose: unbranched alpha-glucose, stains blue with iodine
Image from http://www.cheng.cam.ac.uk/research/groups/polymer/RMP/nitin/Amylose1.jpg
Image from http://www.cheng.cam.ac.uk/research/groups/polymer/RMP/nitin/Amylose1.jpg
-amylopectin: alpha-glucose chain with side branches, stains red-purple with iodine
Image from http://www.vivo.colostate.edu/hbooks/pathphys/digestion/basics/amylopectin.gif
Image from http://www.vivo.colostate.edu/hbooks/pathphys/digestion/basics/amylopectin.gif
-the chains fold and pack together into starch grains (keményitőszemcse) - 20% amylose, 79% amylopectin, 1% fatty acids and phosphates
GLYCOGEN: found in animals and fungi
GLYCOGEN: found in animals and fungi
- alpha-glucose is arranged like amylopectin (branched), but the chains are shorter and more branched.
-glycogen is found particularly in muscle and liver cells.
-human glycogen stores provide enough energy for a few hours.
ii. Structural polysaccharides
Examples:
CELLULOSE
CELLULOSE
- makes up 50% of the plant cell wall
- each molecule is a chain of approximately 10 000 beta-glucose, unbranched
- OH-groups form H-bonds with neighbouring chains to create a lattice
- about 2000 chains mass together to form microfibrils, which are visible under an electron microscope
Image from http://www.botany.utexas.edu/facstaff/facpages/mbrown/newstat/stat38a.jpg
Image from http://www.botany.utexas.edu/facstaff/facpages/mbrown/newstat/stat38a.jpg
- the lattice is linked together by hemicellulose, which is a short polysaccharide
-cellulose is indigestible to animals (we consider it the "fiber" in our diet!)
-used to produce cotton, rayon, cellophane, celluloid and paper
CHITIN
- found in arthropod exoskeletons and fungal cell walls
- long chains of beta-glucose, but on each monomer the OH-group is substituted by a nitrogenous group (NHCOCH3)








No comments:
Post a Comment