They are not really soluble, rather they form colloids
- colloids are about 500nm, which is larger than particles in solution, but smaller than particles in suspension
- colloids exist in a "sol-gel state", whereby sometimes they appear to be liquid and at other times they are jelly-like (much of the material in cytoplasm is colloid)
Proteins contain: C, H, O and N, sometimes S and P
There are an almost limitless number of proteins, which vary between species and are often species-specific (fajlagosok). They determine the characteristics of a species.
Types of Proteins
a. Structural proteins - these form the organism. eg. hair, nails, feathers, etc.
b. Physiological proteins - these carry out functions, examples include:
-enzymes (biocatalysts)
-carrier molecules (szállítómolekulák)
- pigments (eg. various colour molecules in skin and eyes, haemoglobin in red blood cells)
- hormones (chemical messengers)
- contractile material in muscles
- antibodies (disease protection)
** Proteins are rarely stored (only in seeds and eggs). Proteins are only broken down for energy if a living organism is starving.
PROTEIN STRUCTURE
- a protein is a polymer. Its monomers are called amino acids.
Image from http://api.ning.com/files/xO6ybWgUbfFlk7GUXm9d8dfR--U-fUdPOJEtDzVGgDY_/aminoacidstruc.jpg
- some amino acids are basic, others are neutral - this depends on the variable group
- some amino acids are polar and others are apolar - this depends on the variable group
-amino acids are soluble in water, where they form dipolar ions (zwitterion = ikerion), this means they have BOTH acid-base properties, so they have good buffering capacity.
Synthesis of polypeptides
- amino acids attach to each other by condensation to form covalent peptide bonds
2 amino acids condense to form a dipeptide, 3 form a tripeptide and many joined together form a polypeptide.
- if more than 100 amino acids attach together it is considered a protein
- polypeptides (and proteins) are broken down by hydrolysis
*both condensation and hydrolysis require enzymes to occur.
Structure
Primary structure: this is the number and sequence of the amino acids.
*Insulin was the first protein to have its primary structure determined by a researcher named Fred Sanger
Secondary structure: This type of structure is created by H-bonds forming between amino acid monomers
Alpha helix (eg. keratin - a major component of hair and skin)
Image from http://www.bio.miami.edu/~cmallery/150/protein/alpha-helix.jpg
Beta-pleated sheet (eg. silk protein)
Image from http://student.ccbcmd.edu/courses/bio141/lecguide/unit3/viruses/images/betasheet.jpg
-both structures can be found in a single protein.
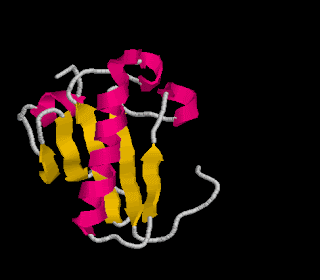
Tertiary structure: This is the secondary structure folded in 3-dimensional space.
-usually forms globular shapes
-bonded by S-bridges (requires the amino acid cysteine), ionic bonds, H-bonds and van der Waals forces
Image from http://lectures.molgen.mpg.de/ProteinStructure/Levels/tertiary.gif
Quaternary structure: A protein has quaternary structure if it is formed of 2 or more subunits (polypeptides). They are held together by various forces including hydrophobic interactions, H-bonds and ionic bonds.
eg. Haemoglobin
Image from http://www.theironfiles.co.uk/images/Haemoglobin_Structure.jpg
Proteins can further be catagorized as simple or complex. A simple protein contains only amino acids, complex proteins often include other elements, such as the iron containing haeme molecule found in haemoglobin (above).
Protein Stability and Denaturation
A protein will be stable (maintain its shape and function) if the environment it is in is appropriate. The most common environmental factors that will cause a protein to denature (lose its shape and/or function) are temperature and pH levels. Some proteins have a wide range of tolerance (can function at 4C and at 40C), while others have a very narrow range. This is a protein-specific characteristic. An example of protein denaturation is when we cook an egg. The white of the egg is almost entirely made of the protein albumin. At room temperature it is a clear liquid. If we increase the temperature, the protein starts to denature (lose its shape and therefore function too) and it become solid and white. Denaturation occurs because the bonds between the amino acids are broken.
Sometimes denaturation is permanent (like cooking an egg), other times it can be reversible.