Generalized animal cell
Generalized plant cell
Image from: http://www.uvm.edu
Structure and function of the cellular organelles:
1. Cytoplasm: the matrix in which all cell organelles and inclusions (insoluble waste or storage products) are found. Made up of the cytosol and cytoskeleton.
-cytosol: 90% water, site of many biochemical reactions
-cytoskeleton: made up of microtubules and microfilaments (string-like proteins). Important in supporting the cell, giving its shape and some cell movements (like extension and contraction of pseudopods in amoeba!), as well as moving organelles within the cell and cell division. The microtubules form parts of the cilia and flagella.
 |
 |
2. Nucleus: control center of most cellular activities. It is surrounded by the nuclear envelope, which contains pores though which materials (proteins and RNA) can pass. The nucleoplasm is found within the envelope, it is similar to the cytoplasm. The nucleolus is the dark patch in the nucleus where ribosomal RNA is formed. Chromatin (DNA and associated proteins) is found around the nucleolus.
3. Endoplasmic reticulum (ER): It is a series of interconnected, flattened sacs, tubes and channels formed by the phospholipid bilayer. There are 2 types: rough ER and smooth ER. Rough ER appears bumpy because it is covered with ribosomes. Its function is to isolate, store and transport proteins produced by the ribosomes. The smooth ER produces and stores lipids.
4. Golgi apparatus: also made of phospholipid bilayers (or membranes), it functions to modify, sort and package macromolecules (like proteins and lipids) for cell secretion (exocytosis) or use within the cell. Imagine it is like a post office; it packages and labels items and sends them to different parts of the cell.
5. Ribosomes: make proteins from amino acids. They can be found attached to the ER or floating freely in the cytoplasm. They are made of rRNA and protein.
In the following picture, the connection between the various cell parts can be observed. Information about how to build a protein comes from the nucleus as mRNA. It attaches to the ribosome, which "reads" the mRNA and builds a protein from amino acids. The protein is released into the rough ER, then it is packaged in a vesicle (this is a little membrane-bound bubble) and transported to the Golgi apparatus, where it may be modified, packaged into a new vesicle and then transported to the cell surface and released (exocytosis).
Image from: http://scienceblogs.com/transcript/upload/2006/07/ergolgi.jpg
6. Mitochondria: small membrane-bound organelles in the cytoplasm. They are considered the "powerhouse" of the cell as this is where the reactions occur that create ATP (cellular respiration) that provide the cell with energy.
Image from: http://imagineannie.files.wordpress.com/2009/11/mitochondria1.jpg
Image from: http://math.etsu.edu/symbiosis/mitochondria.jpg
7. Chloroplasts: Found only in plants and plant-like protists. They are the site of photosynthesis and contain the green pigment, chlorophyll (along with other, less visible pigments!)
Image from: http://www.scsc.k12.in.us/SMS/Teachers/Martin/chloroplast.jpg
Image from: http://botit.botany.wisc.edu/images/130/Photosynthesis/Chloroplast_EN.gif
8. Lysosomes and peroxisomes: Special vacuoles, which are designed to digest things (such as worn-out or excess cell parts, food particles, invading viruses or bacteria, etc) with digestive enzymes. They contain different digestive enzymes, therefore digest different things. Lysosomes are formed from the Golgi apparatus, whereas peroxisomes are formed from the ER.
9. Cell wall: It is found in plant, algal and fungal cells. It is completely permeable, but it contains cellulose (plants and algae) or chitin (fungus), which protects and supports the cell.
10. Cell membrane: It is a phospholipid bilayer. It is both elastic and rigid and helps give the cell its shape. It is selectively permeable, thus helps control transport of substances and homeostasis. It is permeable to gases and water, but many substances can only pass through it with the help of proteins, such as channels, protein pumps and receptor proteins.
Image from: http://macro.magnet.sfu.edu/cells/plasmamembrane/images/plasmamembranefigure1.jpg
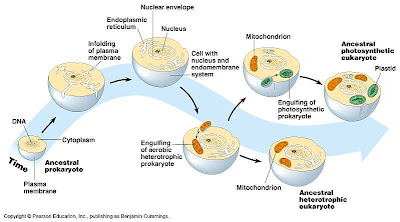
The Endosymbiotic Theory
- proposed by Lynn Margulis at the end of the 1960s, it was initially ridiculed, but is now well-accepted.
- suggests that certain prokaryotic cells engulfed (endocytosed) other cells, but instead of being digested, these cells continued to live and each provided the other with some benefits (symbiosis).
- mitchondria: an anaerobic bacteria engulfed a smaller aerobic bacteria. Mutual advantages? The aerobic bacteria provided the larger, anaerobic bacteria with the ability to survive in areas with oxygen, while the larger bacteria ingested food and provided protection to the smaller aerobic one.
- chloroplast: a photosynthetic bacteria was engulfed by a larger anerobic bacteria. Advantages? I am sure you can figure these out for yourselves!!
- evidence: These organelles are surrounded by a double lipid bilayer (their own, plus the one of the cell that engulfed them!). These organelles also have their own DNA and divide independently of the cells that they are found in.
Image from: http://www.origin-of-mitochondria.net/?attachment_id=120