BUT, they do dissolve in apolar solvents, like alcohols, ethers, etc.
Lipids include:
Fats and oils
Phospholipids
Streroids
Carotenoids
Waxes
A. Neutral fats and oils
- these have high energy content
-structure: made of a backbone with adjoining fatty acid chains
the backbone is an alcohol, commonly glycerol,
Image from lhs2.lps.org/staff/sputnam/Biology/U2Biochemistry/lipid_lab.htm
Fatty acids: there is considerable variation in fatty acids, but basically they are hydrocarbon chains. If all the bonds are single, then it is saturated, because it contains the maximum possible H's. It will form a fat (solid).
The general formula is CH3(CH2)nCOOH.
CH3 is the methyl group.
(CH2)n is the variable chain, for example if n=16, then it is a stearic acid chain (common in adipose tissue - zsírszövet).
COOH is the carboxyl group.
If the chain contains double bonds, then it is unsaturated. It will form an oil (liquid).
Cells produce fats and oils by condensing (see lecture 1) a glycerol and 3 fatty acids. These molecules are also called triglycerides.
Image from http://www.biology-books.com/Standard/ch2/16.jpg
The fatty acid part forms long apolar "tails", which repel water, so are hydrophobic.
Function: energy storage - found in adipose (also called fat) tissue (animals) and storage parenchyma (oily plant seeds). Yeild 38kJ/g, therefore they are very high in energy.
Additional functions: heat insulation, mechanical protection, waterproofing, solvents for vitamins A,D,E and K.
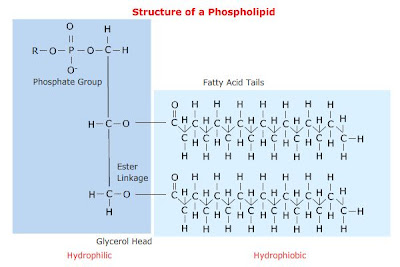
B. Phospholipids
- structurally similar to above, BUT phosphoric acid (foszforsav) takes the place of 1 fatty acid.
Image from http://telstar.ote.cmu.edu/biology/MembranePage/images/phospholipid.jpg
This change creates a hydrophilic "head" region of the molecule and the 2 fatty acid chains form a hydrophobic tail.
http://www.bioteach.ubc.ca/Bio-industry/Inex/graphics/phospholipid.gif
Hence, phospholipids are very important in the formation of plasma membranes of cells, where they form a phospholipid bilayer, with the hydrophobic tails pointing towards each other and the hydrophilic heads in contact with the surroundings.
Image from http://micro.magnet.fsu.edu/cells/plasmamembrane/images/plasmamembranefigure1.jpg
C. Steroids
-insoluble in water
-the basic structure is a sterane skeleton to which various side groups attach.
Image from http://upload.wikimedia.org/wikipedia/commons/thumb/8/88/Steran_num_ABCD.svg/220px-Steran_num_ABCD.svg.png
Some examples of steroids include:
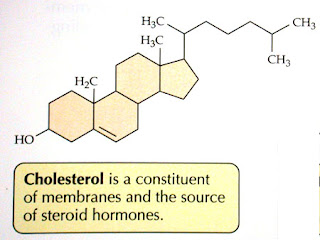
Cholesterol (important for plasma membrane rigidity)
Image from http://academic.brooklyn.cuny.edu/biology/bio4fv/page/cholesterol.JPG
Bile acids (important in lipid digestion)
Sex hormones (estrogen, testosterone) and other steroid hormones
Image from http://helios.hampshire.edu/~msbNS/ns121/images/estrogen.gif
Testosterone
Phytosterols (in plants)
D. Carotenoids
- have conjugated double bonds (the single and the double bonds alternate), which makes them coloured (pigments)
-pigments are longer chains, volatile oils are shorter chains
Examples include:
Vitamin A
Carotene (orange)
Image from http://home.caregroup.org/clinical/altmed/interactions/Images/Nutrients/vitAbetac.gif
Licopene (red)
Xanthophyll (yellow)
E. Waxes
- formed by fatty acids and an alcohol that is larger than glycerol
- important for waterproofing in plants
- found on arthropod exoskeletons (waterproofing)
- wax for bee hives













No comments:
Post a Comment